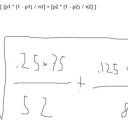Anahid nos muestra una excelente sesión de estadística, donde trataron el tema del error estándar.…
Cálculos de entalpías por cambios de temperatura y estado
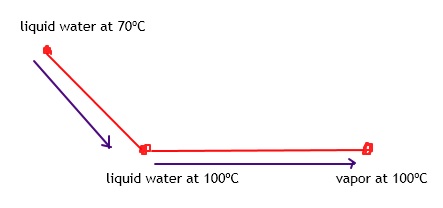
Student’s question: If delta Hvap of water is 40.7 kJ/mol, how much heat is needed to vaporize 25 g. of water? q=n*delta(Hvap)=56.5? —— I got this step. I just don’t know what to do for the next problem. Repeat for T(initial)=70 degrees C
¿Cómo deberíamos empezar la sesión? – La pregunta inicial del estudiante deja ver que ya él ha hecho parte del trabajo y desea revisar si su trabajo es correcto para continuar con el siguiente ejercicio.
Tutor: Hi! How are you today?
Student: Good.
Tutor: Alright, let’s see your question.For the first part, you got it right.
What you think it should be the unit for 56.5?
If it’s heat.
Es importante nunca hacer pregunta sueltas, sino darle una indicación indirecta al estudiante de cómo podría saber la respuesta. El método intuitivo hace que el estudiante se sienta más seguro y comprenda mejor el concepto.
Student: kJ
Tutor: Excellent.
Now, let’s see the second part.
The calculation you did is valid when we have liquid water at 100ºC.
Student: I don’t know what to do because we don’t know the value of c and we don’t have the final temperature of t to find delta t.
Tutor: Alright, then let’s see what’s the process.
We start with water at 70ºC, right?
Student: Yes.
Tutor: If we want to vaporize it, we need to heat to the boiling point, right?
Student: Yes.
Tutor: Good.
Now that we reach the boiling point, we can vaporize it.
While water is vaporized, there is no change in temperature.
At the end we will get vapor at 100ºC.
Student: Oh I get it!
So delta T would be 30 C.
Tutor: Exactly!
En este punto, el principal propósito del tutor es hacer que el estudiante comprenda el concepto básico asociado al problema y que sea el estudiante quien deduzca la información.
Student: But you have to change it to Kelvin, right?
Tutor: Well, since we are making a delta, there’s no need to do it.
Student: Okay.
How do we find C?
Tutor: It’s given in books or tables.
It only depends on the substance.
Student: Oh!
Tutor: The value for water is 4.18J/g ºC.
Constantes y valores de la literatura pueden ser dados al estudiante. También puede dársele al estudiante fuentes de internet donde puede consultar fácilmente la información.
Student: Okay. So then you multiply 4.18, 30, and 25.
Tutor: Exactly!
Student: Okay, then why do you have to add it to n*delta(Hvap)?
Tutor: Because the process requires both steps.
Heating water and vaporizing it.
Student: Okay.
Tutor: However, what would be the unit we get for heat?
Student: J.
Tutor: Exactly!
Student: Why are they different?
Tutor: Because the value for C is given in J.
And Hvap in KJ.
We need to convert one of them.
Student: Yeah, but it doesn’t matter which one you leave it in?
Tutor: Not really.
Student: Okay.
Tutor: Do you prefer to convert to J or kJ?
Student: J.
Tutor: Alright.
Student: I got the answer.
I had another problem that I didn’t get.
Tutor: Alright.
Let’s check it.
Aunque es bueno verificar el trabajo del estudiante, muchos de ellos muestran una excelente comprensión del tema. Cuando el tutor lo crea conveniente, se debe revisar o no lo que el estudiante obtiene como respuesta, dependiendo de su comportamiento durante la sesión.
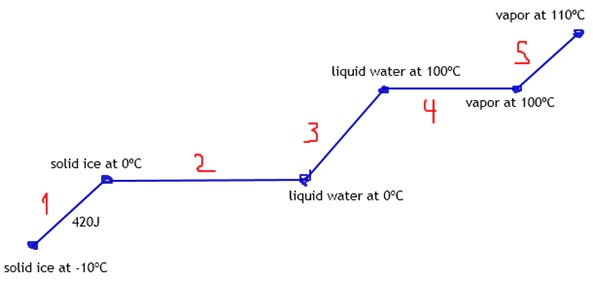
Student: Total q needed to raise T of 20 g ice from -10C to steam at 110C.
Hfus=6.009 kj/mol.
Hvap=40.79 kj/mol.
Cice=2.1 J/g*C.
Csteam=2.08 J/g*C.
Tutor: Alright, any ideas about this one?
Student: Not really.
Tutor: It’s okay.
It’s a bit similar to the former problem.
We start with solid ice at -10ºC.
So, we first need to heat ‘til the melting point, correct?
Student: Yeah.
Tutor: Good.
Student: 100.
Tutor: Let’s notice that’s the boiling point.
The melting point is 0ºC.
Student: Oh, right!
Tutor: So, the next step should be melting ice into liquid water, right?
Student: Yeah.
Tutor: What you think it should be the next process?
Student: Liquid to vapor.
Tutor: But first, getting water at 100ºC.
Since we want to vaporize.
Does it make sense?
Student: Yeah.
Tutor: Good.
Now we can go to vapor, as you mentioned.
Do you think there would be any additional step?
Student: 110ºC.
Would still be vapor?
Tutor: Exactly!
So we heat it.
Each line means we need to calculate a heat.
Student: Okay.
Es importante hacerle un bosquejo de la solución al estudiante cuando éste no está seguro de cómo proceder, así él sabrá qué pasos se seguirán.
Tutor: Any ideas how we can calculate heat number 1?
Student: q=m(c)(delta T).
Tutor: Exactly.
What would be the values?
Student: n would be 20/18.
Delta t would be 10.
What about C?
Tutor: Let’s notice they give C ice.
And it’s given in J/g ºC, so there’s no really need to convert to moles.
We can use grams.
Student: 2.1
Tutor: Right.
Student: Then for line 2, what is the value for C?
Tutor: Let’s notice the horizontal lines are changes of states.
Changes of states don’t use C.
But deltaH.
Student: Oh is that where you use n* delta h?
Tutor: Exactly!
Student: For line 1, I was multiplying the numbers together and I got 23.333, but my teacher got 420 J.
Tutor: Again, remember we don’t need to make 20/18.
C is given in grams.
So, we don’t convert to moles.
It would be just 20 times 2.1 times 10.
Student: Oh, I get it!
Tutor: Great!
Any questions for heat 2?
Student: That would be 20/18*6.009
Tutor: Exactly!
The unit should be J or kJ?
Student: And line 3 would be 20*2.08*100?
kJ.
Tutor: Right.
Remember C for liquid water is 4.18.
Student: Then do you use Hvap for line 4?
Tutor: Exactly.
Student: And line 5, you would use Csteam?
Tutor: Perfect, that’s correct!
Student: Okay. Thank you!
Tutor: No problem
Do you want to check the calculation or do you prefer to do it on your own?
Student: It’s okay. I have the answer because my teacher did. I just didn’t get it when she was explaining…
Tutor: Oh, alright!
Student: *did the problem.
Tutor: Do you have any questions about anything we went over today?
Student: No. I understood it.
Tutor: Great!
Have a nice night!
Student: You too!
Tutor:
Es importante estar seguro de que el estudiante comprendió completamente la solución y puede ahora resolver un problema del mismo tipo por su cuenta.
Colaborador: Alejandro Palacios, Tutor Colombiano de Química